
@ShahidNShah

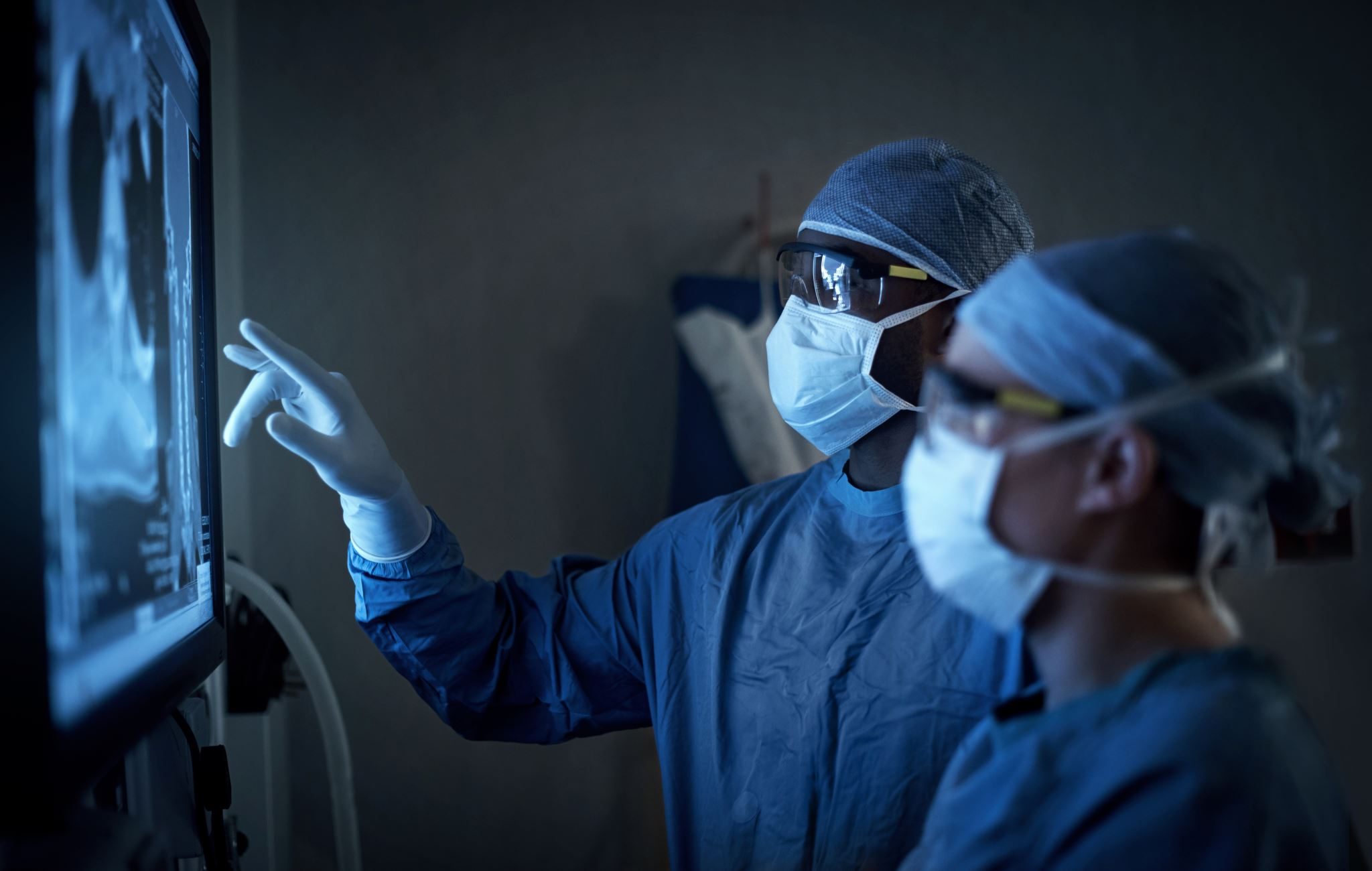
The development of a medical device is a journey marked by innovation, rigorous testing, and strict regulatory oversight. Each step ensures that the final product meets the highest standards of safety and efficacy before it ever reaches a patient. From early-stage prototypes to large-scale manufacturing, the process involves collaboration among engineers, researchers, and medical professionals who work tirelessly to refine each detail. Understanding this lifecycle provides insight into the intricate process that transforms an idea into a life-saving tool, shaping the future of modern healthcare.
Concept and Design Development
Every medical device begins with identifying a need in the healthcare industry. Whether it’s improving an existing technology or introducing an entirely new solution, engineers, researchers, and medical professionals collaborate to develop a concept that addresses specific clinical challenges. The design phase involves creating detailed schematics, selecting appropriate materials, and using advanced technologies to model prototypes. Many companies leverage the expertise of 3D printing companies to produce early-stage prototypes quickly and cost-effectively, allowing for rapid iteration and refinement.
Prototyping and Testing
Once an initial design is established, the prototype undergoes extensive testing to evaluate its functionality, durability, and safety. This phase often includes both mechanical and clinical testing to ensure that the device performs as expected in real-world conditions. Engineers refine materials, adjust configurations, and analyze potential failure points to meet stringent industry standards. Data collected during this stage informs necessary modifications before the device advances to regulatory review.
Regulatory Approval and Manufacturing
Medical devices are subject to rigorous approval processes, varying by country and classification. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), assess clinical data, manufacturing protocols, and overall risk factors before granting approval. Once regulatory clearance is obtained, production moves into large-scale manufacturing. Quality control remains a priority throughout this phase, ensuring that every device produced meets the same strict standards established during testing.
Market Introduction and Clinical Use
After manufacturing, medical devices are distributed to hospitals, clinics, and healthcare providers. Training and education play a crucial role in successful implementation, ensuring that medical professionals understand how to use the device safely and effectively. Continuous monitoring allows manufacturers to track performance, identify potential concerns, and make improvements when necessary. Post-market surveillance is vital for maintaining compliance and patient safety.
End-of-Life Management and Innovation
No medical device lasts forever. Over time, advancements in technology, changes in regulatory guidelines, or new clinical needs may render a device obsolete. Proper disposal, recycling, or refurbishment ensures minimal environmental impact while maintaining ethical and safety considerations. Additionally, insights gained from retired devices often contribute to the next generation of medical innovations, driving further advancements in healthcare technology.
From its initial concept to real-world application, a medical device undergoes an extensive lifecycle designed to prioritize safety, effectiveness, and continuous improvement. The combination of technological advancements, regulatory oversight, and industry expertise ensures that each device contributes meaningfully to patient care, shaping the future of medical innovation. For more information, feel free to look over the accompanying infographic below.

Chief Editor - Medigy & HealthcareGuys.
Booking your first online psychiatry session in Los Angeles can be a big step toward taking care of your mental health. Before diving in, it’s important to know the costs, availability, and …
Posted Feb 18, 2025 Mental Health
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Apr 17, 2025 at 6:07am