
@ShahidNShah

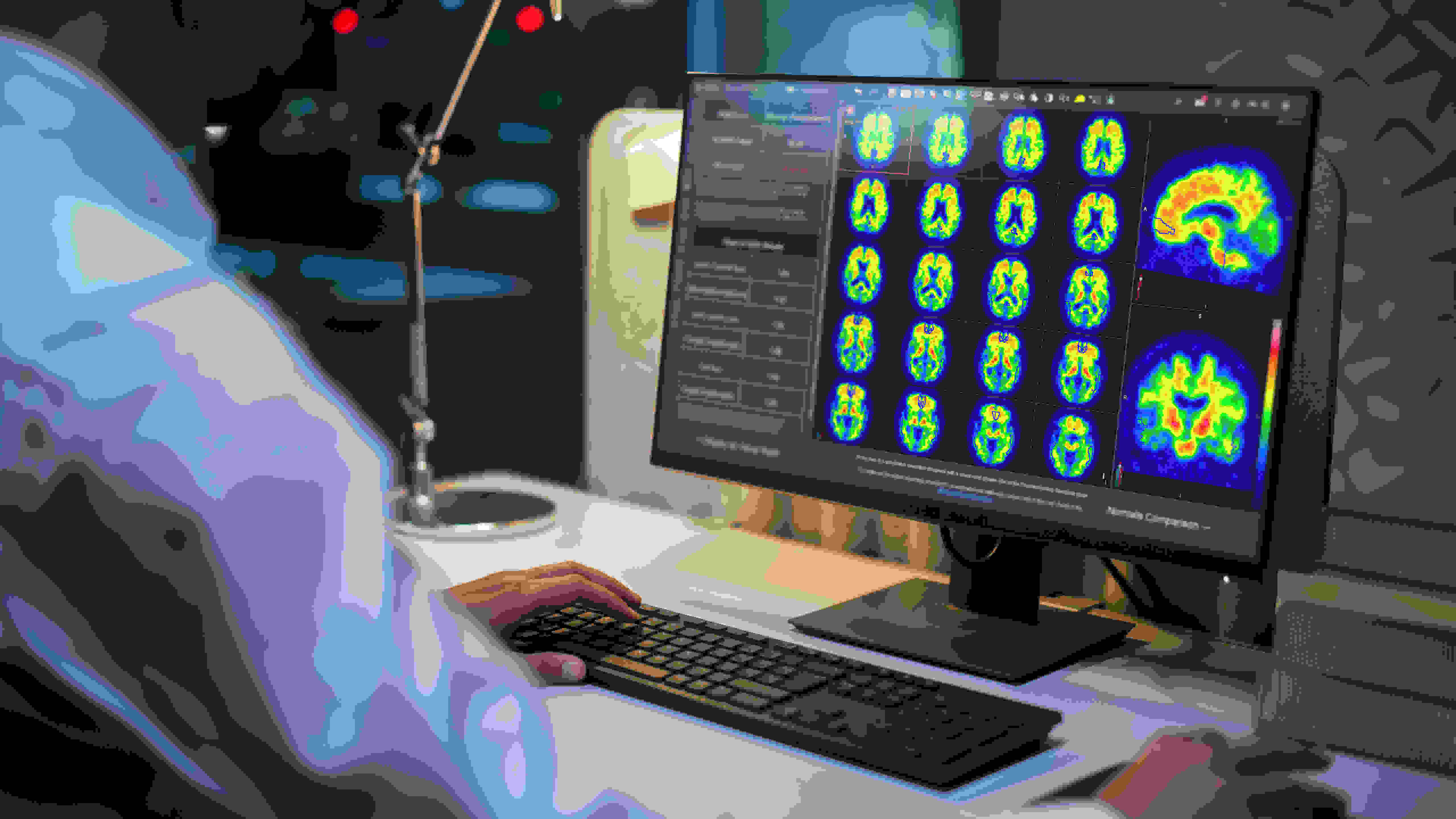
GE HealthCare's MIM Software received FDA clearance for Centiloid scaling in PET imaging for Alzheimer's disease.
– GE HealthCare announced today that its MIM Software has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a groundbreaking tool in the fight against Alzheimer’s disease.
– The advancement allows MIMneuro, a vendor-neutral software solution, to perform “Centiloid scaling” for analyzing and quantifying amyloid plaque density in the brain using positron emission tomography (PET) imaging.
Read on hitconsultant.net
Continue reading at hitconsultant.net
Great Ormond Street Hospital's Dr Shankar Sridharan shares his take on AI application in healthcare and user responsibilities. AI may be optimally applied in healthcare settings without overstepping …
Posted Oct 6, 2024 Healthcare Artificial Intelligence
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Apr 17, 2025 at 6:07am