
@ShahidNShah

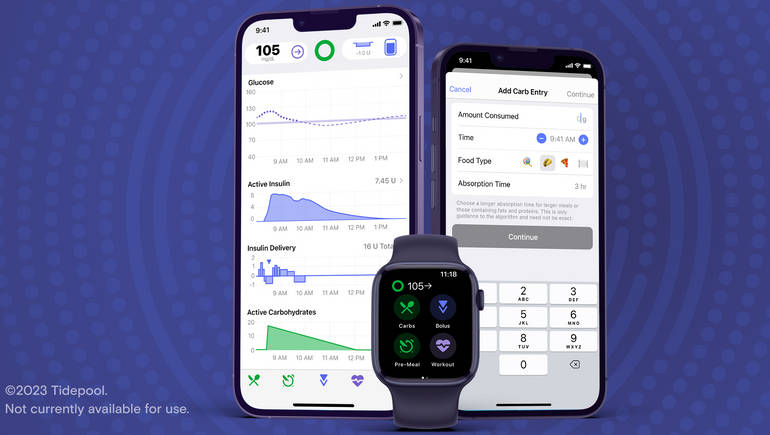
Tidepool Loop, an automated insulin dosing app based on one developed by the DIY diabetes community, received FDA clearance in January. Diabetes sufferers and researchers have been collaborating for years to create an artificial pancreas that can monitor blood sugar levels and deliver insulin as necessary. In the multibillion-dollar market for diabetic devices, those efforts have resulted in the production of the first hybrid closed-loop systems, where continuous glucose monitors and insulin pumps collaborate. These methods nevertheless have certain drawbacks. For starters, since the software that enables the two devices to function together is now built into insulin pumps, patients cannot pick and choose which devices they want to connect together. The regulatory landscape for clinical decision-making software is intricate. Take control of the chaos with the help of this webinar featuring Advarra's device research expertise.
Continue reading at medtechdive.com
Patients often feel uneasy with resident doctors due to sleep deprivation, lack of attentiveness, and the belief that doctors are not interchangeable, leading to concerns about the quality of their …
Posted Apr 14, 2023 Patient Care Healthcare
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Apr 17, 2025 at 6:07am