
@ShahidNShah

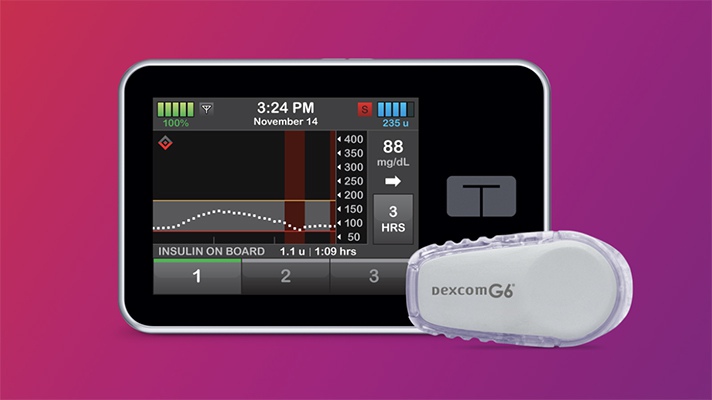
Last week the FDA granted its first marketing authorization to an interoperable insulin pump that is compatible with automated insulin dosing systems, continuous glucose monitors, blood glucose meters and other diabetes therapy devices built by different manufacturers.
The Tandem Diabetes Care t:Slim X2 insulin pump was reviewed through the De Novo premarket review pathway, making it the first of a new medical device category called Alternate Controller Enabled (ACE) infusion pumps. Intended for use by both adults and children with diabetes, the digital device automatically receives insulin dosing commands from a diabetes management device once connected, or can independently infuse insulin when it is on its own.
“Diabetes is a complicated disease that requires close monitoring and carefully tailored treatments,” FDA Comissioner Dr. Scott Gottlieb said in a statement. “We’ve heard from the patient community that having the ability to customize their own diabetes management devices is important to them. Advances in digital health make more tailored approaches to diabetes care possible.”
Continue reading at mobihealthnews.com
As the use of artificial intelligence tools and robotics continues to grow, it’s crucial for organizations to assess the potential security risks posed, says attorney Stephen Wu. Among the …
Posted Feb 19, 2019airobotics
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Apr 21, 2025 at 5:57am