
@ShahidNShah

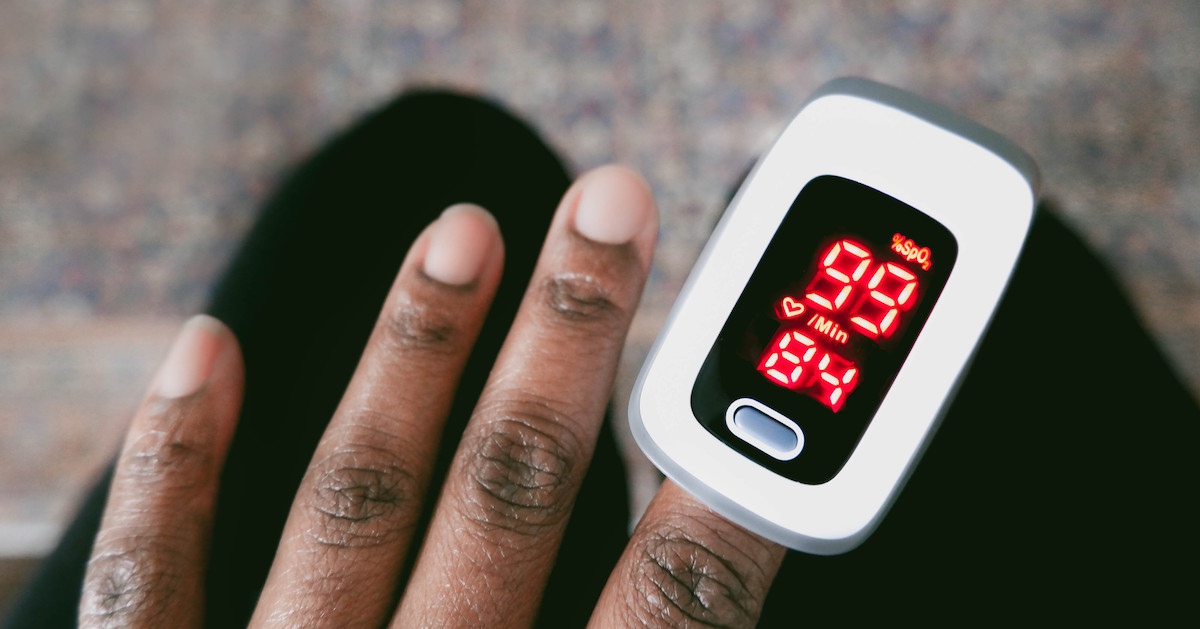
The U.S. Food and Drug Administration is seeking public comment on new recommendations for nonclinical and clinical performance testing to support premarket submissions for pulse oximeters for medical purposes. These include devices with a function that estimates the amount of oxygen in arterial blood and pulse rate.In 2023, the FDA issued a warning about potential risks of inaccuracy under certain circumstances and said it became aware that pulse oximeters could be less accurate in people with dark skin pigmentation. The agency then scheduled public meetings last year to reassess its existing pulse oximetry guidance. The new proposed update, posted on its website, sharpens recommendations for gathering real-world and laboratory clinical data to evaluate device performance accuracy across the range of skin pigmentations, using both subjective and objective methods to standardize evaluations of pulse oximeter products used in healthcare delivery.
Research published in the New England Journal of Medicine in 2020 showed that, after comparing measurements of arterial oxygen saturation with pulse oximetry, Black patients were three times as likely to have occult hypoxemia than white patients.Johns Hopkins School of Medicine has been drawing attention to FDA’s medical device regulation of pulse oximeters, critical of how the agency handled its 510(k) review process in getting these products to the medical, consumer and other industry markets and calling out the agency's reaction time.
Continue reading at healthcareitnews.com
Congress is set to extend telehealth and hospital-at-home flexibilities through a bipartisan continuing resolution, garnering strong support from healthcare groups. The proposed bill includes a …
Connecting innovation decision makers to authoritative information, institutions, people and insights.
Medigy accurately delivers healthcare and technology information, news and insight from around the world.
Medigy surfaces the world's best crowdsourced health tech offerings with social interactions and peer reviews.
© 2025 Netspective Foundation, Inc. All Rights Reserved.
Built on Apr 28, 2025 at 12:53pm